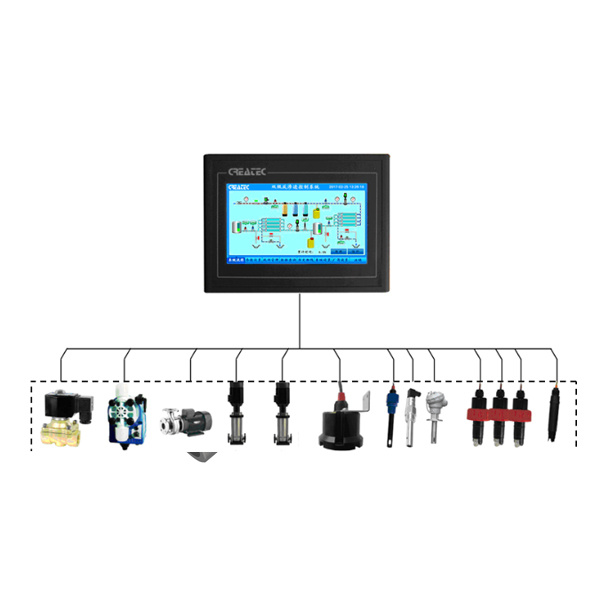
Table of Contents
Importance of Calibration in ph meter Measurements
The ph meter is a crucial tool in various industries, including food and beverage, pharmaceuticals, and environmental monitoring. It measures the acidity or alkalinity of a solution by determining the concentration of hydrogen ions present. However, for accurate and reliable measurements, calibration of the ph meter is essential.
Calibration is the process of adjusting the ph meter to ensure that it provides accurate and precise readings. This is done by comparing the ph meter readings to known standard solutions with a defined pH value. By calibrating the ph meter, any potential errors or deviations in the readings can be identified and corrected.
One of the main reasons why calibration is important in ph meter measurements is to maintain the accuracy of the instrument. Over time, pH meters can drift or become less accurate due to factors such as electrode aging, contamination, or improper handling. By calibrating the ph meter regularly, these issues can be detected and corrected, ensuring that the instrument provides reliable measurements.
Another reason why calibration is crucial in ph meter measurements is to ensure consistency and reproducibility of results. In industries where precise pH measurements are critical, such as in pharmaceutical manufacturing or water treatment plants, even small errors in pH readings can have significant consequences. By calibrating the ph meter, operators can be confident that the measurements are accurate and consistent, allowing for reliable decision-making and quality control.
Calibration also helps to identify and correct any systematic errors in the ph meter. Systematic errors are errors that occur consistently in the same direction, leading to biased measurements. By calibrating the ph meter with standard solutions of known pH values, operators can determine if there are any systematic errors present and make the necessary adjustments to correct them.
In addition to maintaining accuracy and consistency, calibration of the ph meter is also important for compliance with regulatory requirements. In industries such as food and beverage or pharmaceuticals, there are strict guidelines and standards that govern the quality and safety of products. Regular calibration of the ph meter is often a requirement to ensure that the measurements meet these standards and regulations.
To calibrate a ph meter, operators typically use two or more standard solutions with known pH values. These solutions are usually prepared using certified reference materials and are traceable to national standards. By immersing the ph meter electrode into the standard solutions and adjusting the instrument to match the pH values, operators can calibrate the ph meter and ensure its accuracy.

In conclusion, calibration is a critical aspect of ph meter measurements. It helps to maintain the accuracy, consistency, and reliability of the instrument, identify and correct systematic errors, and ensure compliance with regulatory requirements. By calibrating the ph meter regularly with standard solutions, operators can be confident in the accuracy of their pH measurements and make informed decisions based on reliable data.
Understanding the Nernst Equation in ph meter Operation
The ph meter is a widely used instrument in various fields such as chemistry, biology, and environmental science. It is used to measure the acidity or alkalinity of a solution, which is crucial in many scientific experiments and industrial processes. The operation of a ph meter is based on the Nernst equation, which describes the relationship between the measured pH value and the voltage generated by the electrode system.
The Nernst equation is named after the German physical chemist Walther Nernst, who formulated it in the early 20th century. It is a fundamental equation in electrochemistry that relates the electrode potential of an electrochemical cell to the concentration of ions in the solution. In the case of a ph meter, the Nernst equation is used to calculate the pH of a solution based on the voltage generated by the pH electrode.
The Nernst equation for a pH electrode is given by:
E = E0 + (0.05916/n) * log([H+])
Where:
E is the measured electrode potential
E0 is the standard electrode potential
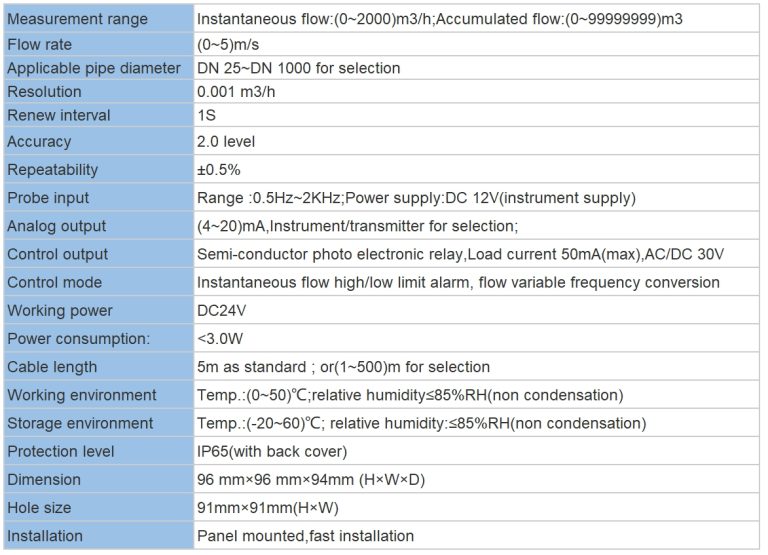
| Model | DO-810/1800 dissolved oxygen meter |
| Range | 0-20.00 mg/L |
| Accuracy | \u00b10.5% FS |
| Temp. Comp. | 0-60\u2103 |
| Oper. Temp. | 0\uff5e60\u2103 |
| Sensor | dissolved oxygen sensor |
| Display | Segment code operation/128*64 LCD Screen(DO-1800) |
| Communication | Optional RS485 |
| Output | 4-20mA output\u00a0 High/Low limit double relay control |
| Power | AC 220V\u00b110% 50/60Hz or AC 110V\u00b110% 50/60Hz or DC24V/0.5A |
| Working Environment | Ambient temperature:0\uff5e50\u2103 |
| Relative humidity\u226485% | |
| Dimensions | 96\u00d796\u00d7100mm(H\u00d7W\u00d7L) |
| Hole Size | 92\u00d792mm(H\u00d7W) |
| Installation Mode | Embedded |
n is the number of electrons transferred in the redox reaction
[H+] is the concentration of hydrogen ions in the solution
In the case of a ph meter, the standard electrode potential (E0) is determined by the construction of the electrode and the reference electrode used in the measurement. The number of electrons transferred (n) in the redox reaction is usually 1 for a pH electrode, as it involves the exchange of one hydrogen ion. The concentration of hydrogen ions ([H+]) in the solution is what we are trying to measure with the ph meter.
The Nernst equation is essential for the accurate operation of a ph meter. It allows us to convert the voltage generated by the electrode system into a meaningful pH value that can be used in scientific experiments and industrial processes. By understanding the Nernst equation, we can appreciate the underlying principles of pH measurement and ensure the reliability of ph meter readings.
In conclusion, the Nernst equation plays a crucial role in the operation of a ph meter. It allows us to convert the electrode potential into a pH value, which is essential for measuring the acidity or alkalinity of a solution. By understanding the Nernst equation, we can ensure the accuracy and reliability of ph meter readings in various scientific and industrial applications.