“Accurate measurements, precise results – the science of pH meters.”
Table of Contents
Principles of pH Measurement
A ph meter is a crucial tool in various industries, including agriculture, food and beverage production, pharmaceuticals, and environmental monitoring. It is used to measure the acidity or alkalinity of a solution, providing valuable information about the chemical composition of the substance being tested. Understanding how a ph meter works is essential for obtaining accurate and reliable results.

At the heart of a ph meter is a glass electrode, which is sensitive to hydrogen ions in a solution. When immersed in a liquid, the glass electrode generates a voltage that is proportional to the concentration of hydrogen ions present. This voltage is then converted into a pH value by the meter, which is displayed on a digital screen.
To ensure accurate measurements, the glass electrode must be properly calibrated before each use. This is done by immersing the electrode in a buffer solution with a known pH value and adjusting the meter accordingly. Calibration is essential for maintaining the accuracy and reliability of the ph meter.
When using a ph meter, it is important to properly prepare the sample being tested. The solution should be stirred to ensure uniform distribution of ions and bubbles should be removed to prevent interference with the measurement. It is also important to rinse the electrodes with distilled water between measurements to avoid contamination.
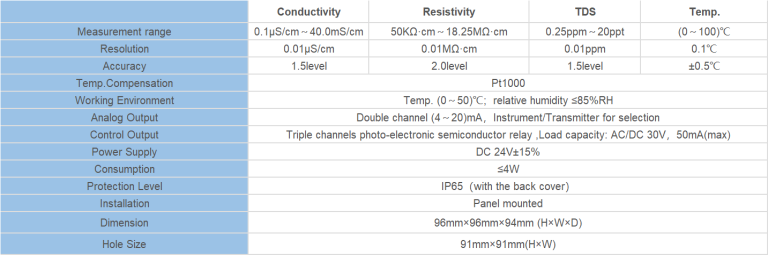
| Model | pH/ORP-3500 pH/orp meter |
| Range | pH:0.00~14.00 ; ORP: (-2000~+2000)mV; Temp.:(0.0~99.9)°C (Temp.Compensation: NTC10K) |
| Resolution | pH:0.01 ; ORP: 1mV; Temp.:0.1°C |
| Accuracy | pH:+/-0.1 ; ORP: +/-5mV(electronic unit); Temp.: +/-0.5°C |
| Temp. compensation | Range: (0~120)°C; element: Pt1000 |
| Buffer Solution | 9.18; 6.86; 4.01; 10.00; 7.00; 4.00 |
| Medium Temp. | (0~50)°C (with 25°C as standard) manual/automatic temp. compensation for selection |
| Analog output | Isolated one Channel(4~20)mA, Instrument/Transmitter for selection |
| Control Output | Double relay output (single contact ON/OFF) |
| Working Environment | Temp.(0~50)℃; relative humidity <95%RH (non-condensing) |
| Storage Environment | Temp.(-20~60)℃;Relative Humidity ≤85%RH (none condensation) |
| Power Supply | DC 24V; AC 110V; AC220V |
| Power consumption | <3W |
| Dimension | 48mmx96mmx80mm(HxWxD) |
| Hole Size | 44mmx92mm(HxW) |
| Installation | Panel mounted, fast installation |
One of the key factors that can affect the accuracy of pH measurements is temperature. pH meters are typically calibrated at a specific temperature, so it is important to take into account any temperature variations when making measurements. Some pH meters have built-in temperature compensation features to account for temperature changes and provide more accurate results.
In addition to measuring the pH of a solution, pH meters can also be used to monitor changes in pH over time. This is particularly useful in processes where pH plays a critical role, such as in fermentation or chemical reactions. By continuously monitoring pH levels, operators can make adjustments to ensure optimal conditions for the desired outcome.
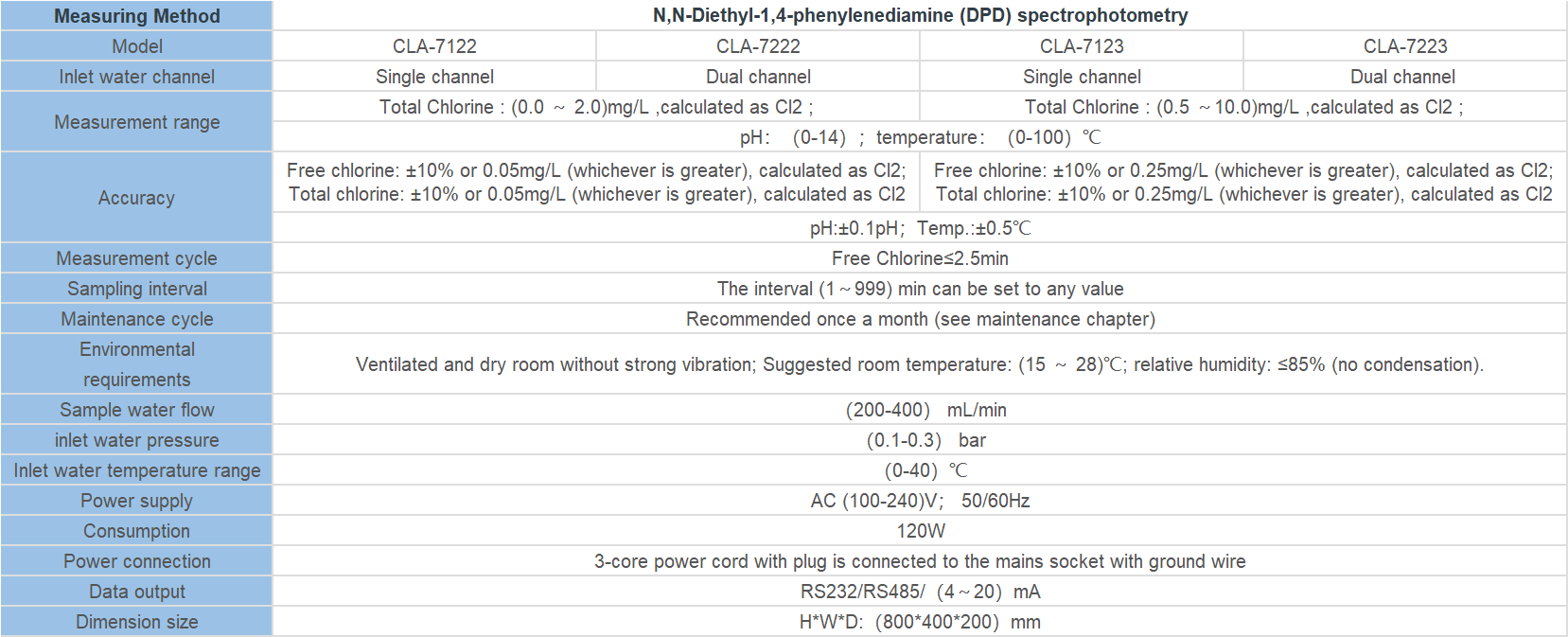
| Controller type | ROC-7000 Single-stage/Double-stage Reverse osmosis control integrated system | |||||
| Cell constant | 0.1cm-1 | 1.0 cm-1 | 10.0cm-1 | |||
| Conductivity measurement parameters | Raw water conductivity | (0~2000) | (0~20000) | |||
| Primary conductivity | (0~200) | (0~2000) | ||||
| Secondary conductivity | (0~200) | (0~2000) | ||||
| Temperature compensation | Automatic compensation on the basis of 25 ℃ ,compensation range(0~50)℃ | |||||
| Accuracy | Matched precision:1.5 level | |||||
| Flow measurement range | Instantaneous flow | (0~999)m3/h | ||||
| Accumulative flow | (0~9999999)m3 | |||||
| pH | Measurement range | 2-12 | ||||
| measurement parameters | Accuracy | ±0.1pH | ||||
| Temperature compensation | Automatic compensation on the basis of 25 ℃ ,compensation range(0~50)℃ | |||||
| DI acquisition | Input signal | Low pressure switch of Tap water,high level of pure water tank, low level of pure water tank, low pressure switch before the pump, high pressure switch after the primary booster pump,high level of secondary pure water tank, low level of secondary pure water tank,high pressure switch after the secondary booster pump | ||||
| Signal Type | Passive switch contact | |||||
| DO Control | Control output | Inlet valve, primary flush valve, primary drain valve, antiscalant pump, raw water pump, primary booster pump, secondary booster pump, secondary flush valve, secondary drain valve, pH adjustment metering pump. | ||||
| Electrical contact | Relay(ON/OFF) | |||||
| Load capacity | 3A(AC 250V)~ 3A(DC 30V) | |||||
| Display screen | Screen color:TFT;resolution:800×480 | |||||
| Working power | Working power | DC 24V±4V | ||||
| Power consumption | ≤6.0W | |||||
| Working environment | Temperature:(0~50)℃;Relative humidity:≤85%RH(non condensation) | |||||
| Storage environment | Temperature:(-20~60)℃;Relative humidity:≤85%RH(non condensation) | |||||
| Installation | Panel mounted | Hole(Length×Width,192mm×137mm) | ||||
Overall, understanding how a ph meter works is essential for obtaining accurate and reliable measurements. By following proper calibration procedures, preparing samples correctly, and taking into account factors such as temperature, users can ensure that their ph meter provides accurate and consistent results. pH meters play a crucial role in a wide range of industries, and their accurate measurements are essential for ensuring product quality, safety, and compliance with regulations.
Understanding pH Electrodes and Calibration
A ph meter is a crucial tool in various industries, including agriculture, food and beverage production, and environmental monitoring. It measures the acidity or alkalinity of a solution by detecting the concentration of hydrogen ions present. Understanding how a ph meter works is essential for accurate measurements and reliable results.
At the heart of a ph meter is the pH electrode, which is a specialized sensor designed to detect changes in hydrogen ion concentration. The electrode consists of a glass membrane that is sensitive to pH changes and a reference electrode that provides a stable voltage for comparison. When the electrode is immersed in a solution, the glass membrane selectively allows hydrogen ions to pass through, generating a voltage that is proportional to the pH of the solution.
To ensure accurate measurements, pH meters must be calibrated regularly using buffer solutions with known pH values. Calibration adjusts the meter’s readings to match the actual pH of the solution being tested. During calibration, the ph meter is first immersed in a buffer solution with a known pH value, typically pH 7.0 for neutral solutions. The meter is then adjusted to match the pH value of the buffer solution by using the calibration controls.
In addition to calibration, proper maintenance of the pH electrode is essential for accurate measurements. The electrode should be rinsed with distilled water before and after each use to remove any contaminants that may affect its performance. It should also be stored in a storage solution to keep the glass membrane hydrated and prevent it from drying out.
When using a ph meter, it is important to handle the electrode with care to avoid damaging the delicate glass membrane. The electrode should be gently immersed in the solution being tested, taking care not to touch the sides or bottom of the container. Stirring the solution gently can help ensure a uniform pH reading.
In some cases, the pH electrode may become dirty or contaminated, leading to inaccurate readings. Cleaning the electrode with a mild detergent or a specialized cleaning solution can help restore its performance. It is important to follow the manufacturer’s instructions for cleaning and maintenance to avoid damaging the electrode.
In conclusion, understanding how a ph meter works is essential for accurate pH measurements. The pH electrode plays a crucial role in detecting changes in hydrogen ion concentration, while calibration ensures that the meter’s readings are accurate and reliable. Proper maintenance and handling of the pH electrode are also important for obtaining accurate results. By following these guidelines, users can ensure that their ph meter provides accurate measurements for a wide range of applications.