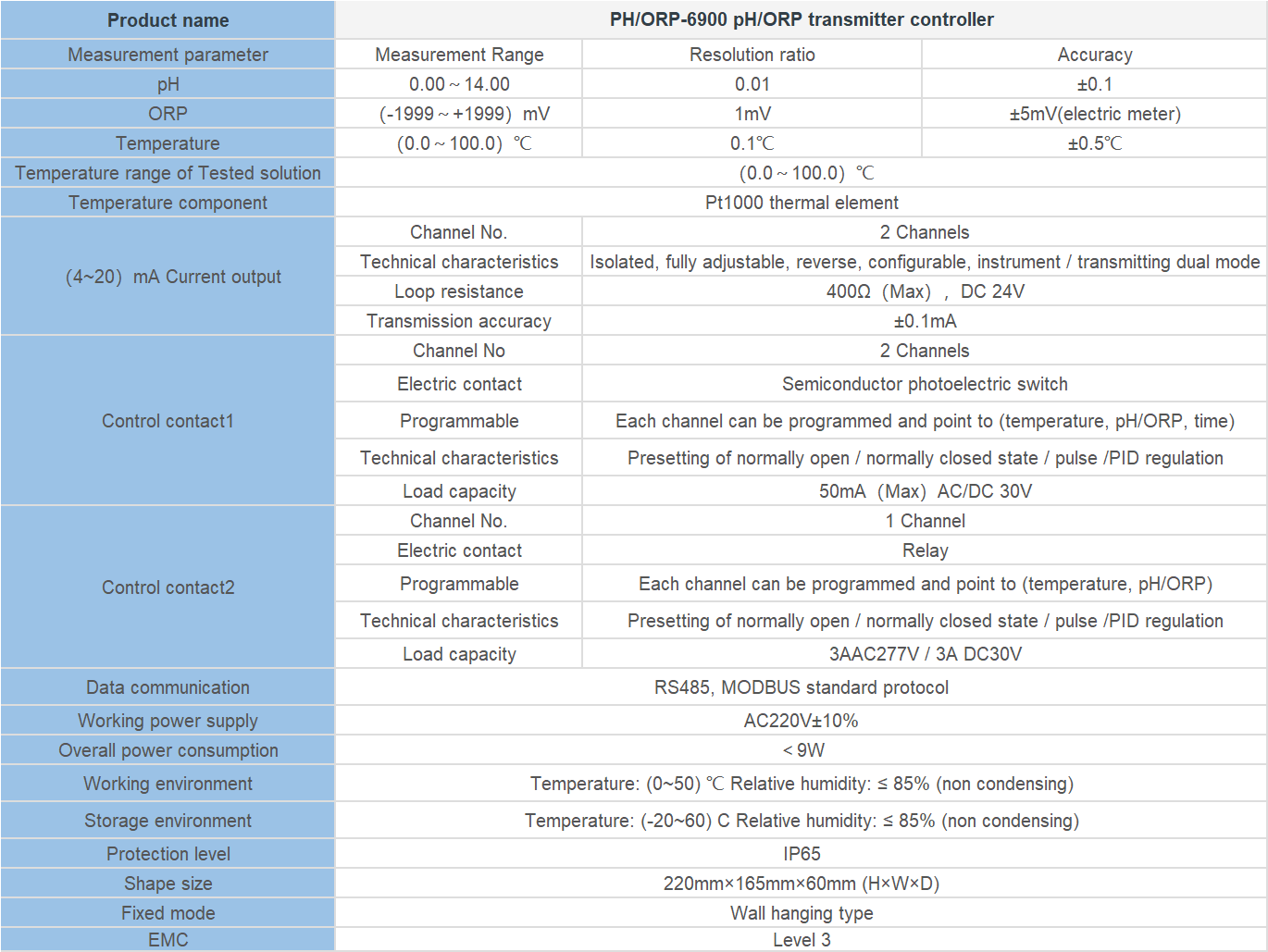
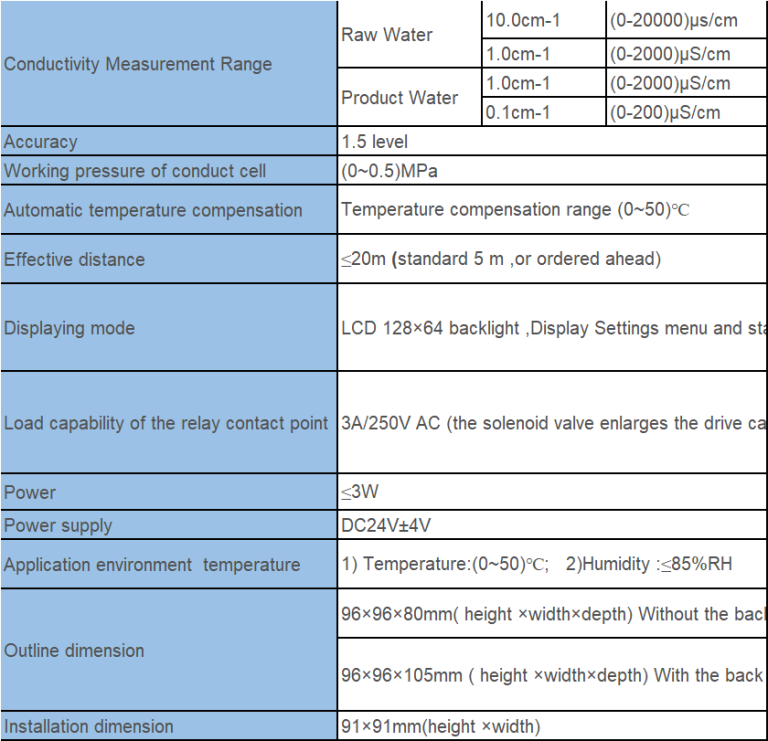
“Measuring more than just pH – exploring conductivity with precision.”
Can a ph meter Measure Conductivity?
A ph meter is a commonly used instrument in laboratories and various industries to measure the acidity or alkalinity of a solution. It works by measuring the concentration of hydrogen ions in a solution and providing a numerical value known as the pH. However, some people may wonder if a ph meter can also measure conductivity, which is the ability of a solution to conduct electricity.
| Model | CL-810/9500 Residual Chlorine Controller |
| Range | FAC/HOCL:0-10 mg/L, ATC TEMP:0-50℃ |
| Accuracy | FAC/HOCL:0.1 mg/L, ATC TEMP:0.1℃ |
| Oper. Temp. | 0~50℃ |
| Sensor | Constant Pressure Residual Chlorine Sensor |
| Waterproof Rate | IP65 |
| Communication | Optional RS485 |
| Output | 4-20mA output; High/Low limit double relay control |
| Power | CL-810:AC 220V±10% 50/60Hz or AC 110V±10% 50/60Hz or DC24V/0.5A |
| CL-9500:AC 85V-265V±10% 50/60Hz | |
| Working Environment | Ambient temperature:0~50℃; |
| Relative humidity≤85% | |
| Dimensions | CL-810:96×96×100mm(H×W×L) |
| CL-9500:96×96×132mm(H×W×L) | |
| Hole Size | 92×92mm(H×W) |
| Installation Mode | Embedded |
While a ph meter is specifically designed to measure the pH of a solution, it is not capable of directly measuring conductivity. Conductivity is a separate property of a solution that is determined by the presence of ions in the solution. These ions can be positively charged (cations) or negatively charged (anions) and are responsible for carrying electrical current through the solution.
To measure conductivity, a separate instrument known as a conductivity meter or conductivity probe is used. This instrument works by applying a small electrical current to the solution and measuring the resulting electrical conductivity. The conductivity of a solution is directly related to the concentration of ions present in the solution, with higher ion concentrations leading to higher conductivity.

In some cases, the conductivity of a solution can also affect the pH measurement obtained from a ph meter. This is because the presence of ions in a solution can influence the behavior of the electrodes in the ph meter, leading to inaccuracies in the pH measurement. In such cases, it may be necessary to account for the conductivity of the solution when interpreting the pH measurement.
| Model | pH/ORP-3500 pH/orp meter |
| Range | pH:0.00~14.00 ; ORP: (-2000~+2000)mV; Temp.:(0.0~99.9)°C (Temp.Compensation: NTC10K) |
| Resolution | pH:0.01 ; ORP: 1mV; Temp.:0.1°C |
| Accuracy | pH:+/-0.1 ; ORP: +/-5mV(electronic unit); Temp.: +/-0.5°C |
| Temp. compensation | Range: (0~120)°C; element: Pt1000 |
| Buffer Solution | 9.18; 6.86; 4.01; 10.00; 7.00; 4.00 |
| Medium Temp. | (0~50)°C (with 25°C as standard) manual/automatic temp. compensation for selection |
| Analog output | Isolated one Channel(4~20)mA, Instrument/Transmitter for selection |
| Control Output | Double relay output (single contact ON/OFF) |
| Working Environment | Temp.(0~50)℃; relative humidity <95%RH (non-condensing) |
| Storage Environment | Temp.(-20~60)℃;Relative Humidity ≤85%RH (none condensation) |
| Power Supply | DC 24V; AC 110V; AC220V |
| Power consumption | <3W |
| Dimension | 48mmx96mmx80mm(HxWxD) |
| Hole Size | 44mmx92mm(HxW) |
| Installation | Panel mounted, fast installation |
Overall, while a ph meter is not capable of directly measuring conductivity, it can still be a valuable tool in conjunction with a conductivity meter for analyzing the properties of a solution. By measuring both pH and conductivity, researchers and scientists can gain a more complete understanding of the composition and behavior of a solution.
In conclusion, a ph meter is a valuable instrument for measuring the acidity or alkalinity of a solution, but it is not capable of directly measuring conductivity. Conductivity is a separate property of a solution that is determined by the presence of ions, and it requires a separate instrument for measurement. However, by using both a ph meter and a conductivity meter together, researchers can obtain a more comprehensive analysis of a solution and gain valuable insights into its properties.