“pH balance: the key to unlocking optimal ORP levels.”
Table of Contents
The Impact of pH on ORP Measurements
pH, or the measure of acidity or alkalinity of a solution, is a crucial factor in many chemical and biological processes. One area where pH plays a significant role is in oxidation-reduction potential (ORP) measurements. ORP is a measure of the tendency of a solution to gain or lose electrons, which can provide valuable information about the overall health and stability of a system. In this article, we will explore the impact of pH on ORP measurements and how changes in pH can affect the accuracy and reliability of these measurements.
When discussing the relationship between pH and ORP, it is important to understand the underlying principles at play. ORP is a measure of the electron activity in a solution, while pH is a measure of the concentration of hydrogen ions. These two factors are closely related, as changes in pH can directly impact the availability of electrons in a solution. In general, a lower pH corresponds to a higher electron activity, while a higher pH corresponds to a lower electron activity.
One way in which pH can affect ORP measurements is through the presence of acidic or alkaline substances in a solution. Acids are substances that release hydrogen ions when dissolved in water, leading to a decrease in pH. Alkaline substances, on the other hand, release hydroxide ions, which can increase the pH of a solution. These changes in pH can alter the electron activity in a solution, leading to fluctuations in ORP measurements.

For example, in a solution with a low pH (high acidity), there are more hydrogen ions present, which can compete with other ions for electrons. This can lead to a higher electron activity and a more positive ORP reading. Conversely, in a solution with a high pH (low acidity), there are fewer hydrogen ions available, which can result in a lower electron activity and a more negative ORP reading.
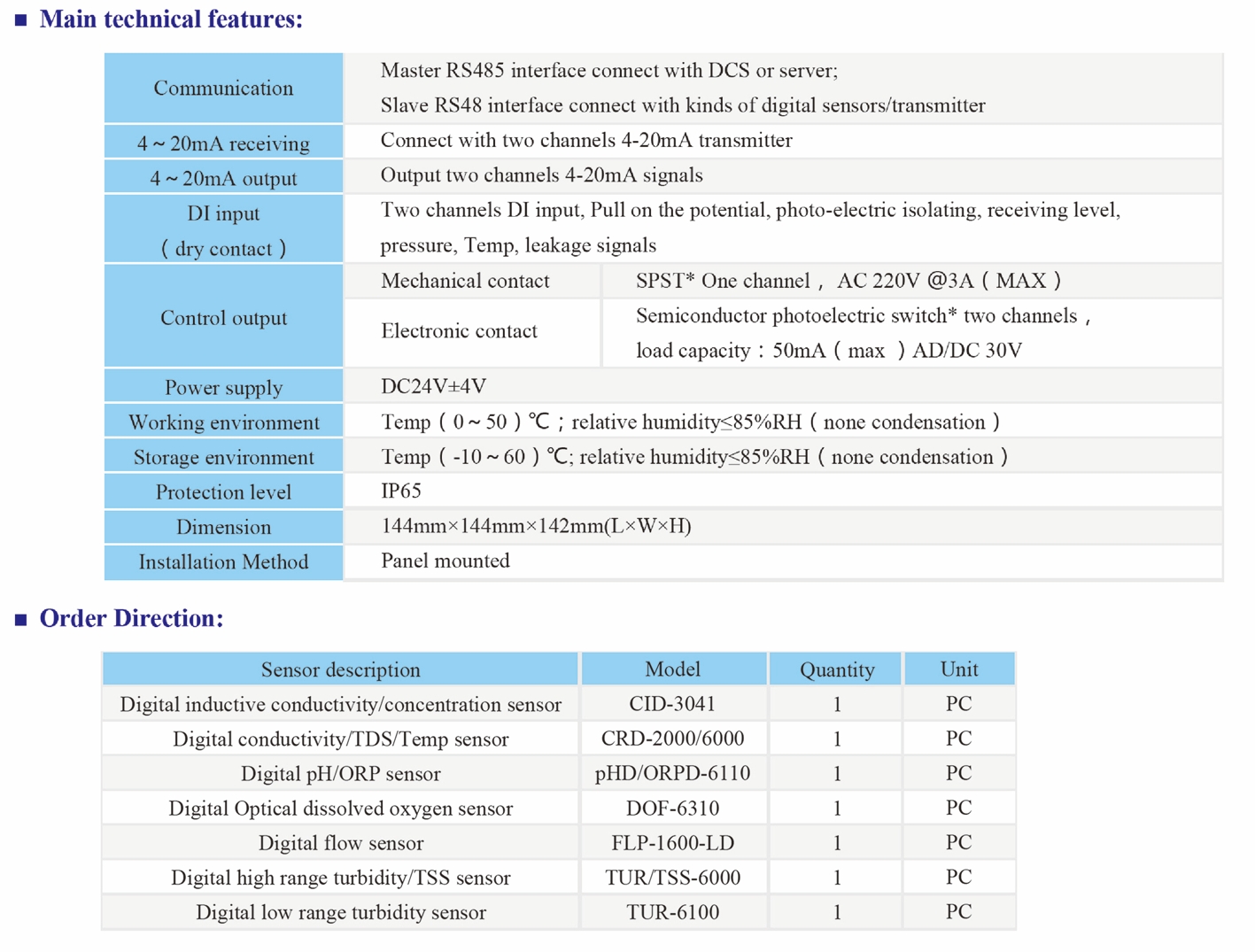
| Model | EC-8851/EC-9900 High Precision Conductivity/resistivity controller |
| Range | 0-200/2000/4000/10000uS/cm |
| 0-20/200mS/cm 0-18.25MΩ | |
| Accuracy | Conductivity:1.5%; Resistivity:2.0%(FS) |
| Temp. Comp. | Automatic temperature compensation based on 25℃ |
| Oper. Temp. | Normal 0~50℃; High temp 0~120℃ |
| Sensor | 0.01/0.02/0.1/1.0/10.0cm-1 |
| Display | LCD Screen |
| Current Output | 4-20mA output/2-10V/1-5V |
| Output | High/Low limit dual relay control |
| Power | DC24V/0.5A or |
| AC85-265V±10% 50/60Hz | |
| Working Environment | Ambient temperature:0~50℃ |
| Relative humidity≤85% | |
| Dimensions | 96×96×72mm(H×W×L) |
| Hole Size | 92×92mm(H×W) |
| Installation Mode | Embedded |
It is important to note that the relationship between pH and ORP is not always straightforward. Other factors, such as temperature, pressure, and the presence of other chemicals, can also influence ORP measurements. However, pH remains a key factor that must be considered when interpreting ORP data.
In practical terms, the impact of pH on ORP measurements means that changes in pH can lead to inaccuracies in ORP readings. For example, if a solution undergoes a sudden change in pH due to the addition of an acidic or alkaline substance, the ORP reading may not accurately reflect the true electron activity in the solution. This can have implications for a wide range of industries, including water treatment, food and beverage production, and environmental monitoring.
To mitigate the effects of pH on ORP measurements, it is important to carefully calibrate and maintain ORP sensors. Regular calibration ensures that the sensor is accurately measuring electron activity, regardless of changes in pH. Additionally, it is important to monitor and control pH levels in a solution to minimize fluctuations that could impact ORP readings.
In conclusion, pH plays a significant role in ORP measurements, as changes in pH can directly impact the electron activity in a solution. Understanding the relationship between pH and ORP is essential for accurate and reliable measurements in a variety of industries. By carefully calibrating sensors and monitoring pH levels, the impact of pH on ORP measurements can be minimized, ensuring that data is both accurate and meaningful.
How pH Levels Influence ORP Readings
pH, or potential of hydrogen, is a measure of the acidity or alkalinity of a solution. It is a crucial factor in many chemical and biological processes, as it can influence the behavior of various substances in a solution. One question that often arises is whether pH affects ORP, or oxidation-reduction potential. In this article, we will explore how pH levels can influence ORP readings and why it is important to consider both factors when analyzing a solution.
To understand the relationship between pH and ORP, it is essential to first grasp the concept of ORP. ORP is a measure of the tendency of a solution to gain or lose electrons, which is a key indicator of its ability to undergo oxidation or reduction reactions. In simpler terms, it is a measure of the solution’s overall redox potential. ORP readings are typically expressed in millivolts (mV), with positive values indicating an oxidizing environment and negative values indicating a reducing environment.
The pH of a solution can have a significant impact on its ORP readings. This is because pH influences the distribution of ions in a solution, which in turn affects the redox reactions that take place. In general, as the pH of a solution becomes more acidic (lower pH), the concentration of hydrogen ions (H+) increases, leading to a more oxidizing environment. Conversely, as the pH becomes more alkaline (higher pH), the concentration of hydroxide ions (OH-) increases, resulting in a more reducing environment.
It is important to note that the relationship between pH and ORP is not linear. While changes in pH can influence ORP readings, the extent of this influence can vary depending on the specific solution and the substances present. For example, in some cases, a decrease in pH may lead to a significant increase in ORP, while in others, the effect may be minimal.
| Instrument model | FET-8920 | |
| Measurement range | Instantaneous flow | (0~2000)m3/h |
| Accumulative flow | (0~99999999)m3 | |
| Flow rate | (0.5~5)m/s | |
| Resolution | 0.001m3/h | |
| Accuracy level | Less than 2.5% RS or 0.025m/s.whichever is the largest | |
| Conductivity | >20μS/cm | |
| (4~20)mA output | Number of channels | Single channel |
| Technical features | Isolated,reversible,adjustable, meter/transmission dual mode | |
| Loop resistance | 400Ω(Max), DC 24V | |
| Transmission accuracy | ±0.1mA | |
| Control output | Number of channels | Single channel |
| Electrical contact | Semiconductor photoelectric relay | |
| Load capacity | 50mA(Max), DC 30V | |
| Control mode | Instantaneous amount upper/lower limit alarm | |
| Digital output | RS485(MODBUS protocol ),Impulse output1KHz | |
| Working power | Power supply | DC 9~28V |
| source | Power Consumption | ≤3.0W |
| Diameter | DN40~DN300(can be customized) | |
| Working environment | Temperature:(0~50) ℃; Relative humidity: ≤85%RH(none condensation) | |
| Storage environment | Temperature:(-20~60) ℃; Relative humidity: ≤85%RH(none condensation) | |
| Protection grade | IP65 | |
| Installation method | Insertion pipeline installation | |
One factor that can complicate the relationship between pH and ORP is the presence of buffering agents in a solution. Buffering agents help to maintain the pH of a solution within a certain range by resisting changes in pH when acids or bases are added. In the presence of buffering agents, changes in pH may not have a significant impact on ORP readings, as the buffering agents help to stabilize the redox reactions.
When analyzing a solution, it is essential to consider both pH and ORP readings to gain a comprehensive understanding of its chemical properties. By measuring both parameters, researchers can assess the overall redox potential of a solution and determine how pH influences its oxidative or reductive capabilities.
In conclusion, pH levels can indeed influence ORP readings in a solution. The relationship between pH and ORP is complex and can vary depending on the specific solution and the substances present. By considering both pH and ORP measurements, researchers can gain valuable insights into the redox potential of a solution and its ability to undergo oxidation or reduction reactions.





