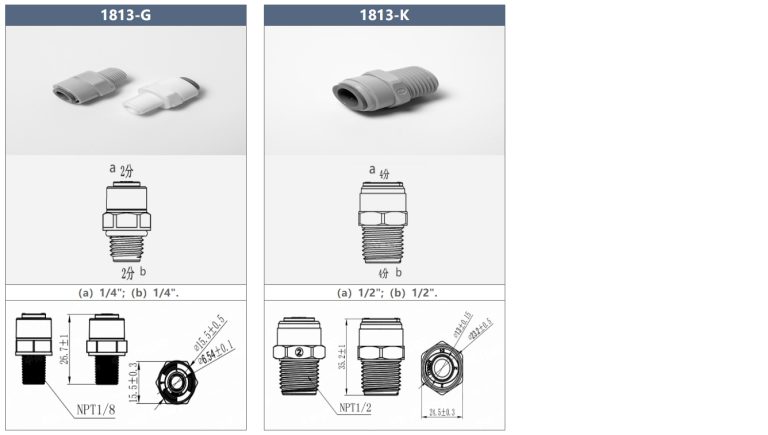
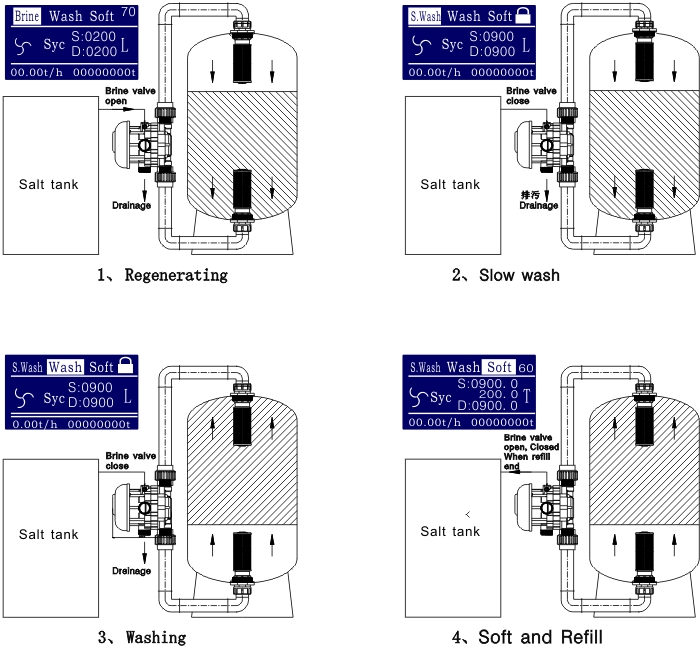
It seems we can’t find what you’re looking for. Perhaps searching can help.

vanne de contrôle de débit Delta P
Comprendre la fonctionnalité et les applications des vannes Delta P de contrôle de débit Les vannes de régulation de débit Delta P, également connues sous le nom de vannes de régulation de pression différentielle (DPCV), font partie intégrante des systèmes fluidiques et jouent un rôle crucial dans la régulation du débit de fluide. Ces vannes…