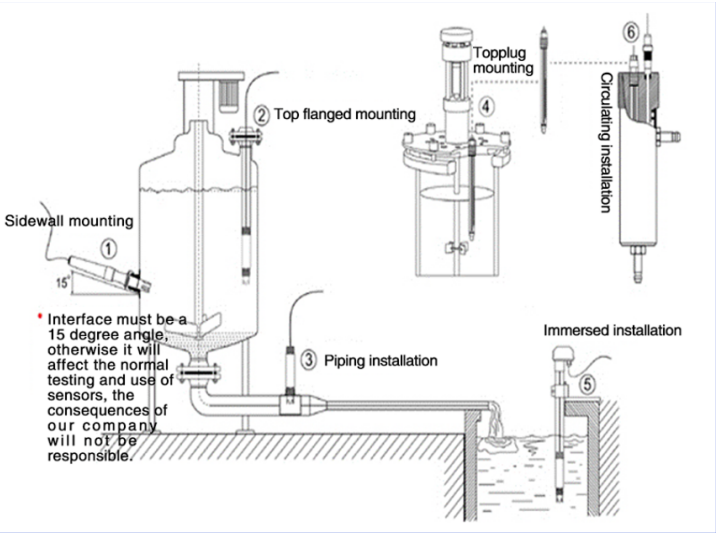
Understanding the Principles of pH Measurement
A ph meter is a crucial tool in the field of chemistry and biology for measuring the acidity or alkalinity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating alkalinity. But how exactly does a ph meter measure the pH of a solution?

The key component of a ph meter is the glass electrode, which is sensitive to hydrogen ions in the solution being tested. When the glass electrode comes into contact with the solution, a potential difference is generated between the inside and outside of the electrode. This potential difference is directly proportional to the concentration of hydrogen ions in the solution, which is used to calculate the pH value.
| CCT-3300 | ||||
| Constant | 10.00cm-1 | 1.000cm-1 | 0.100cm-1 | 0.010cm-1 |
| Conductivity | (500\uff5e20,000) | (1.0\uff5e2,000) | (0.5\uff5e200) | (0.05\uff5e18.25) |
| \u03bcS/cm | \u03bcS/cm | \u03bcS/cm | M\u03a9\u00b7cm | |
| TDS | (250\uff5e10,000) | (0.5\uff5e1,000) | (0.25\uff5e100) | \u2014\u2014 |
| ppm | ppm | ppm | ||
| Medium Temp. | (0\uff5e50)\u2103\uff08Temp. Compensation : NTC10K\uff09 | |||
| Resolution | Conductivity: 0.01\u03bcS/cm\uff1b0.01mS/cm | |||
| TDS: 0.01ppm | ||||
| Temp.: 0.1\u2103 | ||||
| Accuracy | Conductivity:1.5%\uff08FS\uff09 | |||
| Resistivity: 2.0%\uff08FS\uff09 | ||||
| TDS:1.5%\uff08FS\uff09 | ||||
| Temp:\u00b10.5\u2103 | ||||
| Analog Output | Single isolated(4\uff5e20)mA\uff0cinstrument/transmitter for selection | |||
| Control Output | SPDT relay\uff0cLoad Capacity: AC 230V/50A(Max) | |||
| Working Environment | Temp:\u00a0(0\uff5e50)\u2103\uff1bRelative humidity\uff1a\u00a0\u226485%RH(none condensation) | |||
| Storage Environment | Temp:(-20\uff5e60)\u2103; Relative humidity\u00a0\u226485%RH(none condensation) | |||
| Power Supply | DC 24V/AC 110V/AC 220V\u00b115%\uff08for selection\uff09 | |||
| Dimension | 48mm\u00d796mm\u00d780mm (H\u00d7W\u00d7D) | |||
| Hole Size | 44mm\u00d792mm (H\u00d7W) | |||
| Installation | Panel mounted, fast installation | |||
To measure the pH of a solution, the glass electrode is immersed in the solution, and a reference electrode is also placed in the solution to provide a stable reference point for the measurement. The potential difference between the glass electrode and the reference electrode is then measured by the ph meter, and this value is used to calculate the pH of the solution.
One important factor to consider when using a ph meter is calibration. pH meters need to be calibrated regularly using buffer solutions with known pH values to ensure accurate measurements. Calibration adjusts the ph meter to account for any drift or changes in the sensitivity of the glass electrode over time.
Another factor to consider is temperature. The pH of a solution can be affected by temperature, so it is important to measure the temperature of the solution and use a temperature compensation feature on the ph meter to correct for any temperature-related changes in pH.
In addition to the glass electrode and reference electrode, pH meters also contain a display unit that shows the pH value of the solution being tested. Some pH meters also have additional features such as automatic temperature compensation, data logging capabilities, and the ability to store calibration data for multiple buffer solutions.
Overall, a ph meter measures the pH of a solution by using a glass electrode sensitive to hydrogen ions, a reference electrode for stability, and a display unit to show the pH value. Regular calibration and temperature compensation are important factors to consider when using a ph meter to ensure accurate and reliable measurements.
In conclusion, understanding how a ph meter measures the pH of a solution is essential for anyone working in the field of chemistry or biology. By knowing the principles behind pH measurement and how to properly use a ph meter, researchers and scientists can obtain accurate and reliable pH measurements for their experiments and analyses.