It seems we can’t find what you’re looking for. Perhaps searching can help.
katup multiport pentaair tr60
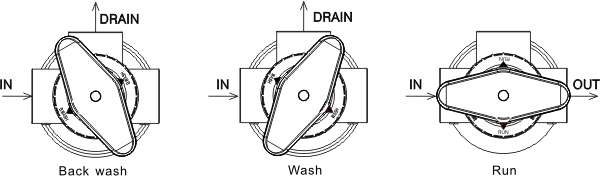
Manfaat Mengupgrade ke Katup Multiport Pentair TR60 Jika Anda memiliki kolam renang, Anda pasti tahu betapa pentingnya memiliki sistem penyaringan yang andal untuk menjaga air kolam Anda tetap bersih dan jernih. Salah satu komponen penting dari sistem penyaringan kolam adalah katup multiport, yang mengontrol aliran air melalui filter dan memungkinkan Anda melakukan tugas pemeliharaan penting…