Table of Contents
Benefits of Using 21 CFR Compliant pH Meters in Pharmaceutical Manufacturing
In the pharmaceutical manufacturing industry, ensuring the accuracy and reliability of pH measurements is crucial for maintaining product quality and safety. pH meters are essential tools used to measure the acidity or alkalinity of a solution, which can have a significant impact on the effectiveness and stability of pharmaceutical products. To meet regulatory requirements and ensure data integrity, many pharmaceutical companies are turning to 21 CFR compliant pH meters.
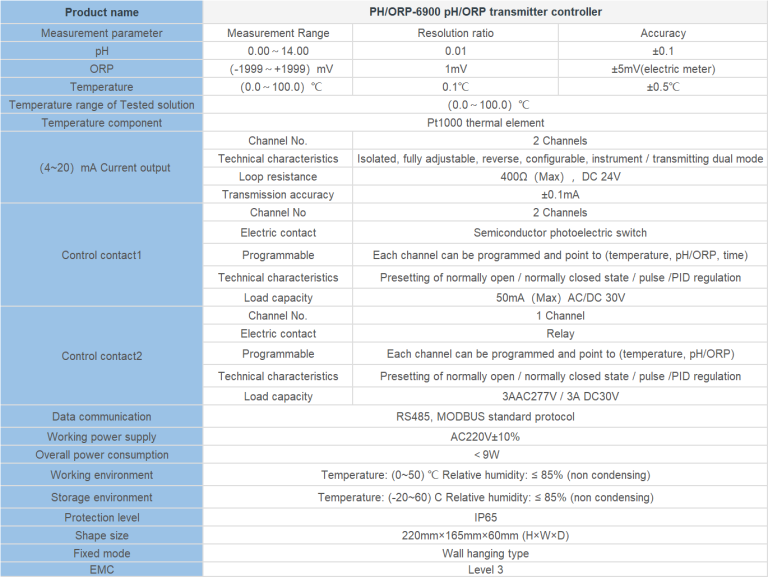
| FL-9900 High Precision Type Runner Flow Controller | ||
| Measuring range | Frequency | 0\uff5e2K Hz |
| Velocity of flow | 0.5\uff5e5 m/s | |
| Instantaneous flow | 0\uff5e2000 m\u00b3/h | |
| Cumulative flow | 0\uff5e9999 9999.999 m\u00b3 | |
| Applicable pipe diameter range | DN15\uff5eDN100;DN125\uff5eDN300 | |
| Resolution | 0.01 m\u00b3/h | |
| Refresh rate | 1s | |
| Accuracy class | Level 2.0 | |
| Repeatability | \u00b10.5% | |
| Sensor input | Radius:0\uff5e2K Hz | |
| Supply voltage:DC 24V(instrument internal supply) | ||
| The electronic unit automatically temperature compensates for errors | +0.5%FS; | |
| 4-20mA | Technical characteristics | Meter/transmitter dual mode (photoelectric isolation) |
| Loop resistance | 500Q(max)\uff0cDC24V; | |
| Transmission accuracy | \u00b10.01mA | |
| Control port | Contact mode | Passive relay control output |
| Load capacity | Load current 5A (max) | |
| Function selection | Instantaneous flow upper/lower alarm | |
| Mains supply | Working voltage: DC24V 4V Power consumption :<; 3.OW | |
| Cable length | Factory configuration: 5m, can be agreed: (1~500) m | |
| Environmental requirement | Temperature: 0~50\u2103; Relative humidity: \u226485%RH | |
| Storage environment | Temperature: (-20~60) \u2103; Humidity: 85%RH | |
| Overall dimension | 96\u00d796\u00d772mm\uff08height \u00d7 width \u00d7 depth\uff09 | |
| Opening size | 92\u00d792mm | |
| Installation mode | Disc mounted, fast fixed | |
| Sensor | Body material | Body: Engineering plastic PP; Bearing :Zr02 high temperature zirconia |
| Flow rate range | 0.5\uff5e5 m/s | |
| Withstand pressure | \u22640.6MPa | |
| Supply voltage | lDC 24V | |
| Output pulse amplitude| | Vp\u22658V | |
| Normal pipe diameter | DN15\uff5eDN100;DN125\uff5eDN600 | |
| Medium characteristic | Single-phase medium\uff080~60\u2103\uff09 | |
| Installation mode | Direct line insertion | |
21 CFR Part 11 is a regulation established by the Food and Drug Administration (FDA) that sets forth guidelines for electronic records and signatures in the pharmaceutical industry. Compliance with 21 CFR Part 11 is essential for pharmaceutical companies to ensure the integrity, authenticity, and confidentiality of electronic records, including pH measurements. By using 21 CFR compliant pH meters, pharmaceutical manufacturers can demonstrate their commitment to quality and regulatory compliance.
One of the key benefits of using 21 CFR compliant pH meters in pharmaceutical manufacturing is the assurance of data integrity. These pH meters are designed to meet the stringent requirements outlined in 21 CFR Part 11, including electronic record keeping, data security, and audit trails. By using 21 CFR compliant pH meters, pharmaceutical companies can ensure that pH measurements are accurate, reliable, and traceable, which is essential for maintaining product quality and safety.
Another benefit of using 21 CFR compliant pH meters is the ability to streamline the validation process. Validation is a critical step in pharmaceutical manufacturing to ensure that equipment, processes, and systems meet regulatory requirements and produce consistent and reliable results. By using 21 CFR compliant pH meters, pharmaceutical companies can simplify the validation process by leveraging pre-configured validation protocols and documentation that meet the requirements of 21 CFR Part 11.
In addition to ensuring data integrity and streamlining validation, 21 CFR compliant pH meters offer advanced features and functionality that can improve efficiency and productivity in pharmaceutical manufacturing. These pH meters are equipped with user-friendly interfaces, intuitive software, and automated data logging capabilities, making it easier for operators to perform pH measurements accurately and efficiently. By using 21 CFR compliant pH meters, pharmaceutical companies can reduce the risk of human error, improve workflow efficiency, and enhance overall productivity.
Furthermore, using 21 CFR compliant pH meters can help pharmaceutical companies reduce the risk of non-compliance and potential regulatory issues. By following the guidelines set forth in 21 CFR Part 11 and using compliant pH meters, pharmaceutical manufacturers can demonstrate their commitment to quality, data integrity, and regulatory compliance. This can help pharmaceutical companies avoid costly fines, penalties, and reputational damage associated with non-compliance.
In conclusion, the benefits of using 21 CFR compliant pH meters in pharmaceutical manufacturing are clear. These pH meters offer data integrity, streamlined validation, advanced features, and regulatory compliance, all of which are essential for maintaining product quality and safety in the pharmaceutical industry. By investing in 21 CFR compliant pH meters, pharmaceutical companies can ensure the accuracy, reliability, and traceability of pH measurements, ultimately leading to improved efficiency, productivity, and regulatory compliance.
How to Ensure Compliance with 21 CFR Regulations When Using pH Meters in the Food and Beverage Industry
In the food and beverage industry, pH meters play a crucial role in ensuring the quality and safety of products. These devices are used to measure the acidity or alkalinity of a substance, which is important for maintaining the desired taste, texture, and shelf life of food and beverages. However, when using pH meters in this industry, it is essential to comply with the regulations set forth by the Food and Drug Administration (FDA) in Title 21 of the Code of Federal Regulations (CFR).
The 21 CFR regulations outline the requirements for the design, construction, and use of pH meters in the food and beverage industry to ensure accuracy, reliability, and consistency in pH measurements. Compliance with these regulations is essential to prevent contamination, ensure product quality, and protect consumer health.
One of the key requirements outlined in 21 CFR for pH meters is calibration. Calibration is the process of adjusting the ph meter to ensure accurate and reliable measurements. According to the regulations, pH meters must be calibrated regularly using standard buffer solutions to verify their accuracy. This helps to ensure that pH measurements are consistent and reliable, which is critical for maintaining product quality and safety.
In addition to calibration, 21 CFR also specifies requirements for the maintenance and cleaning of pH meters. pH meters must be properly maintained and cleaned to prevent contamination and ensure accurate measurements. Regular maintenance, such as replacing electrodes and sensors, is essential to keep pH meters in good working condition and prevent errors in pH measurements.
Another important aspect of compliance with 21 CFR regulations is the documentation of pH measurements. According to the regulations, all pH measurements must be recorded and maintained for a specified period of time. This documentation is important for traceability and quality control purposes, as it allows for the tracking of pH measurements and ensures that any deviations from the desired pH levels are identified and addressed promptly.
Furthermore, 21 CFR also outlines requirements for the storage and handling of pH meters. pH meters must be stored in a clean and dry environment to prevent contamination and damage. They should also be handled with care to avoid any physical damage that could affect their accuracy and reliability.
To ensure compliance with 21 CFR regulations when using pH meters in the food and beverage industry, it is essential to establish a comprehensive quality control program. This program should include regular calibration, maintenance, cleaning, documentation, and storage of pH meters, as well as training for personnel on proper ph meter use and handling.
Overall, compliance with 21 CFR regulations is essential for ensuring the accuracy, reliability, and consistency of pH measurements in the food and beverage industry. By following these regulations and implementing a quality control program, companies can maintain product quality, prevent contamination, and protect consumer health. Failure to comply with these regulations can result in fines, product recalls, and damage to a company’s reputation. Therefore, it is crucial for companies in the food and beverage industry to prioritize compliance with 21 CFR regulations when using pH meters.