The Benefits of Filtering Water to Soften It: Exploring the Science Behind the Process
Water softening is a process that is used to reduce the levels of calcium and magnesium ions in hard water. These ions are responsible for the formation of scale, which can cause damage to plumbing systems and appliances. Softening water can also improve the taste and smell of the water, as well as reduce the amount of soap needed for cleaning.
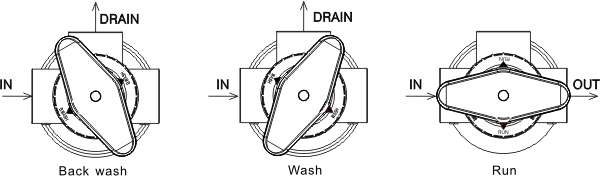
The process of water softening involves the use of a water softener, which is a device that is designed to remove the calcium and magnesium ions from the water. This is done by passing the water through a bed of resin beads, which are charged with sodium ions. As the hard water passes through the resin beads, the calcium and magnesium ions are exchanged for the sodium ions, resulting in softened water.
The benefits of water softening are numerous. Softened water is less likely to form scale, which can cause damage to plumbing systems and appliances. It also reduces the amount of soap needed for cleaning, as soap is more effective in soft water. Additionally, softened water can improve the taste and smell of the water, as well as reduce the amount of soap scum that is left behind after cleaning.
The science behind the process of water softening is relatively simple. The resin beads used in the process are charged with sodium ions, which are attracted to the calcium and magnesium ions in the hard water. As the hard water passes through the resin beads, the calcium and magnesium ions are exchanged for the sodium ions, resulting in softened water.
| Model | Valve Material | Inlet/Outlet | Continuous (0.1Mpa drop) | Peak (0.175Mpa drop) | Cv** | Maximum Backwash (0.175Mpa drop) | Distributor Pilot | Drain Line | Brine Line | Mounting Base | Height (from top of the tank) |
| CM56 | Noryl | 1-3/4″ | 4.54m³/h | 5.9m³/h | 5.2 | 7gpm | 13/16″, 1″(1.05)O.D. | 1/2″(female) | 3/8″ | 2.5″-8NPSM | 7″ |

In conclusion, water softening is a process that is used to reduce the levels of calcium and magnesium ions in hard water. This process can provide numerous benefits, such as reducing the amount of scale that is formed, improving the taste and smell of the water, and reducing the amount of soap needed for cleaning. The science behind the process is relatively simple, as it involves the exchange of calcium and magnesium ions for sodium ions.