“Accurate measurements for precise results – calibrate your ph meter regularly.”
Table of Contents
Importance of Regular Calibration for pH Meters
A ph meter is a crucial tool in various industries, including food and beverage, pharmaceuticals, environmental monitoring, and research laboratories. It measures the acidity or alkalinity of a solution by determining the concentration of hydrogen ions present. However, to ensure accurate and reliable results, it is essential to calibrate the ph meter regularly.
Calibration is the process of adjusting the ph meter to a known standard to ensure its accuracy. Over time, pH meters can drift out of calibration due to factors such as temperature changes, electrode aging, or exposure to harsh chemicals. If a ph meter is not calibrated regularly, it can lead to inaccurate readings, which can have serious consequences in industries where precise measurements are critical.
Regular calibration of a ph meter is essential to maintain its accuracy and reliability. By calibrating the ph meter with standard buffer solutions, you can ensure that it is measuring pH levels correctly. Calibration also helps to identify any drift in the ph meter‘s readings and allows for adjustments to be made to correct any errors.

In addition to ensuring accurate measurements, regular calibration of a ph meter is also important for quality control purposes. In industries such as food and beverage or pharmaceuticals, where pH levels can impact product quality and safety, it is crucial to have confidence in the accuracy of pH measurements. By calibrating the ph meter regularly, you can be confident that your products meet the required standards and regulations.
Furthermore, regular calibration of a ph meter can help to extend its lifespan. By detecting and correcting any issues early on, you can prevent damage to the ph meter and ensure that it continues to provide accurate measurements for an extended period. This can help to save time and money on costly repairs or replacements in the long run.
Calibrating a ph meter is a relatively simple process that can be done in-house by following the manufacturer’s instructions. Most pH meters come with calibration solutions and instructions for calibration, making it easy for users to perform the necessary adjustments. It is recommended to calibrate a ph meter before each use or at least once a day, depending on the frequency of use and the criticality of the measurements.
In conclusion, regular calibration of a ph meter is essential for maintaining accuracy, reliability, and quality control in various industries. By calibrating the ph meter regularly, you can ensure that it is providing accurate measurements, identify any drift in readings, and extend its lifespan. It is a simple yet crucial step that should not be overlooked to ensure the integrity of your pH measurements.
Consequences of Using an Uncalibrated ph meter
A ph meter is a crucial tool in various industries, including agriculture, food and beverage production, pharmaceuticals, and environmental monitoring. It measures the acidity or alkalinity of a solution, providing valuable information for quality control and research purposes. However, to ensure accurate and reliable results, it is essential to calibrate the ph meter regularly.
Calibration is the process of adjusting the ph meter to a known standard to ensure its accuracy. Over time, pH meters can drift out of calibration due to factors such as temperature changes, electrode aging, or exposure to contaminants. Using an uncalibrated ph meter can lead to inaccurate readings, which can have serious consequences in various applications.

In agriculture, pH meters are used to monitor soil acidity levels, which can affect plant growth and nutrient uptake. If a ph meter is not calibrated, farmers may apply the wrong amount of fertilizers or soil amendments, leading to poor crop yields and financial losses. Inaccurate pH measurements can also result in environmental damage, as excess fertilizers can leach into water sources, causing pollution.
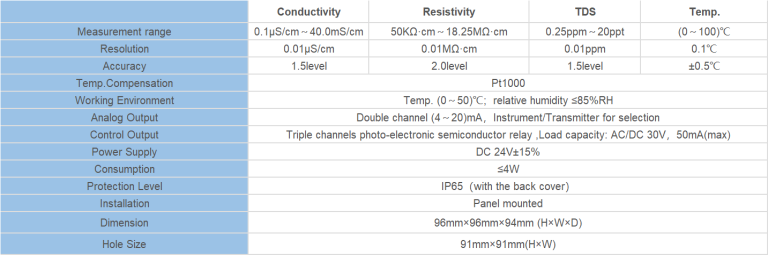
| Model | CM-230S Ecomonical conductivity monitor |
| Range | 0-200/2000/4000/10000uS/cm |
| 0-100/1000/2000/5000PPM | |
| Accuracy | 1.5%(FS) |
| Temp. Comp. | Automatic temperature compensation based on 25℃ |
| Oper. Temp. | Normal 0~50℃; High temp 0~120℃ |
| Sensor | Standard:ABS C=1.0cm-1 (others are optional) |
| Display | LCD Screen |
| Zero Correction | Manual correction for low range 0.05-10ppm Set from ECO |
| Unit Display | uS/cm or PPM |
| Power | AC 220V±10% 50/60Hz or AC 110V±10% 50/60Hz or DC24V/0.5A |
| Working Environment | Ambient temperature:0~50℃ |
| Relative humidity≤85% | |
| Dimensions | 48×96×100mm(H×W×L) |
| Hole Size | 45×92mm(H×W) |
| Installation Mode | Embedded |
In the food and beverage industry, pH meters are used to monitor the acidity of products such as wine, beer, and dairy products. Uncalibrated pH meters can lead to products that are either too acidic or not acidic enough, affecting taste, texture, and shelf life. In extreme cases, incorrect pH measurements can pose health risks to consumers if the product becomes contaminated with harmful bacteria.
In pharmaceuticals, pH meters are used to monitor the acidity of drug formulations and ensure their stability and efficacy. Using an uncalibrated ph meter can result in medications that are either too acidic or too alkaline, leading to reduced effectiveness or potential side effects. Inaccurate pH measurements can also compromise the quality and safety of pharmaceutical products, putting patients at risk.
In environmental monitoring, pH meters are used to assess water quality in lakes, rivers, and oceans. Uncalibrated pH meters can lead to inaccurate measurements of acidity levels, which can impact aquatic ecosystems and wildlife. For example, changes in water pH can affect the survival and reproduction of fish and other aquatic organisms, leading to ecological imbalances and biodiversity loss.
| Model | NTU-1800 Online Turbidity Tester |
| Range | 0-10/100/4000NTU or as required |
| Display | LCD |
| Unit | NTU |
| DPI | 0.01 |
| Accuracy | ±5% FS |
| Repeatability | ±1% |
| Power | ≤3W |
| Power Supply | AC 85V-265V±10% 50/60Hz or |
| DC 9~36V/0.5A | |
| Working Environment | Ambient temperature:0~50℃; |
| Relative humidity≤85% | |
| Dimensions | 160*80*135mm(Hanging) or 96*96mm(Embeded) |
| Communication | 4~20mA and RS-485 communication (Modbus RTU) |
| Switched output | Three-way relay,capacity 250VAC/5A |
Overall, the consequences of using an uncalibrated ph meter can be far-reaching and costly. Inaccurate pH measurements can lead to financial losses, environmental damage, compromised product quality, and potential health risks. To avoid these consequences, it is essential to calibrate pH meters regularly and follow proper maintenance procedures.
Calibrating a ph meter involves using buffer solutions with known pH values to adjust the meter’s readings. This process ensures that the ph meter is accurately measuring the acidity or alkalinity of a solution. By calibrating pH meters regularly, users can ensure the accuracy and reliability of their measurements, leading to better decision-making and improved outcomes in various applications.
In conclusion, calibrating a ph meter is essential to ensure accurate and reliable measurements in agriculture, food and beverage production, pharmaceuticals, and environmental monitoring. Using an uncalibrated ph meter can have serious consequences, including financial losses, environmental damage, compromised product quality, and potential health risks. By following proper calibration procedures and maintenance practices, users can maximize the performance and longevity of their pH meters, leading to better outcomes in their respective fields.